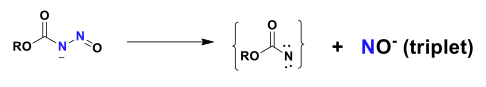
Although a wide range of nitric oxide, NO, and nitrosonium, NO+ donors are known, the number of nitroxyl donors, NO– is very small. More importantly the ones that are available generate singlet or protonated nitroxyl, HNO, rather than the more stable triplet nitroxyl anion, NO–. Proton loss from HNO does generate triplet NO– directly, and instead the singlet NO– from HNO deprotonation is not a source for the more stable triplet anion. Currently there is a real need to understand the properties of triplet NO–, but this has been hampered by the lack of a reliable source We have recently solved this problem by devising a nitrosocarbamate which undergoes a type of Hoffman rearrangement to generate a triplet NO– and a triplet nitrene. In this new project we seek to devise improved NO- donors, ones more stable than the nitrosocarbamates, and to discover new reactions of these reactive species with the aim of understanding its inherent cytotoxicity and possible methods for the clinical detection of its presence in damaged tissues.